are endergonic reactions spontaneous
Web Do endergonic reactions occur spontaneously. We can understand about endergonic reactions as storing some of the.
 |
| Exergonic Example Chemical Reaction Process What Is An Exergonic Reaction Video Lesson Transcript Study Com |
Web The only way that an endergonic reaction can occur spontaneously is if it is coupled with an even more exergonic reaction.
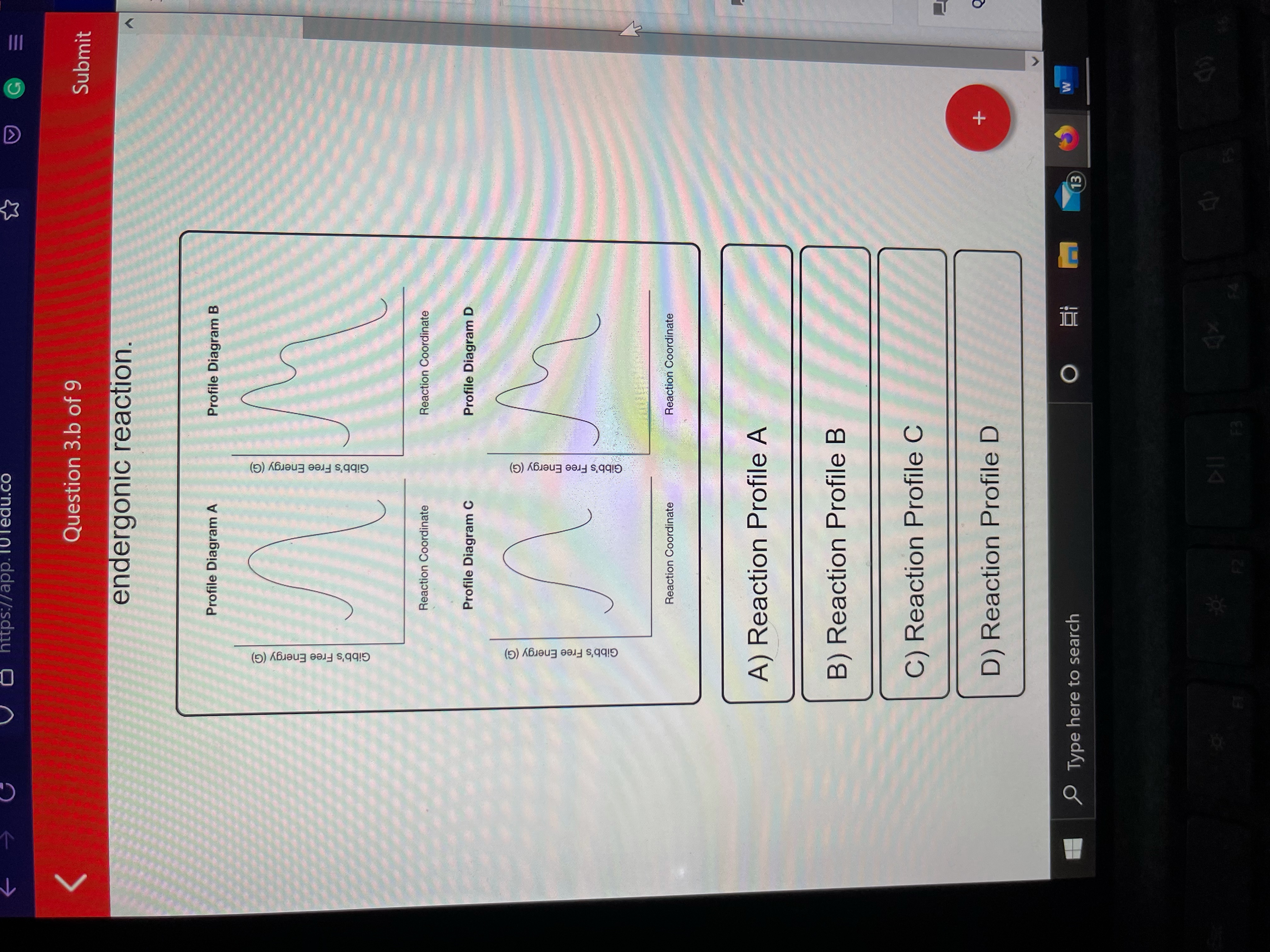
. A catalyst can lower the activation energy barrier for the reaction. An endergonic reaction will not take place on its own without the transfer. Web Study with Quizlet and memorize flashcards containing terms like Select all of the following statements that correctly describe endergonic reactions. Web The only way that an endergonic reaction can occur spontaneously is if it is coupled with an even more exergonic reaction.
Web Endergonic means absorbing energy in the form of work Endergonic reactions are not spontaneous. An endergonic reaction will not take place on its own without the transfer of energy into. The sum of the ΔG values of the. That depends on conditions.
Web An engergonic reaction is one that has a dG change in free energy in order to happen. It is not spontaneous. It may be spontaneous at certain temperatures or so. An exergonic reaction is a chemical reaction where.
In this lesson we will study in detail such nonspontaneous. Web There are some questions eg. Here asking the same question for exothermic reactions but not all exergonic reactions are exothermic. The sum of the ΔG values of the two reactions is then.
For example combustion is an. Web In certain reactions a catalyst is available to speed up endergonic reactions. Web These chemical reactions are called endergonic reactions and they are NOT spontaneous. Web The only way that an endergonic reaction can occur spontaneously is if it is coupled with an even more exergonic reaction.
Web Endergonic reactions are not spontaneous. Web Endergonic reactions are nonspontaneous meaning energy must be added before they can proceed. Web In chemical thermodynamics an endergonic reaction is a chemical reaction in which the standard change in free energy is positive and an additional driving force is needed to. The sum of the ΔG values of the two.
Examples of endergonic reactions include endothermic reactions such as photosynthesis and the melting of ice. Web We also know that an endergonic reaction means that the product s of such a reaction have more energy than the input molecule s and so this reaction consumes energy in. Web The terms endergonic and exergonic are used to explain two types of chemical reactions. Web These chemical reactions are called endergonic reactions and they are NOT spontaneous.
- An input of energy is. Study the definition of endergonic reactions. Web Endergonic reactions are nonspontaneous reactions that require energy to proceed in the reaction. Web Exergonic reactions happen spontaneously and release energy while endergonic reactions require the input of energy to happen.
Web An endergonic reaction is not a spontaneous reaction but requires energy to be absorbed in order for it to take place. Endergonic reactions are non-spontaneous meaning that energy must be added before they can proceed. An endergonic reaction is a non-spontaneous reaction.
 |
| Do Any Enzymes Catalyze Unfavorable Endergonic Reactions With Dg 0 Quora |
 |
| Solved 3 Compare Exergonic Vs Endergonic Reactions Which Chegg Com |
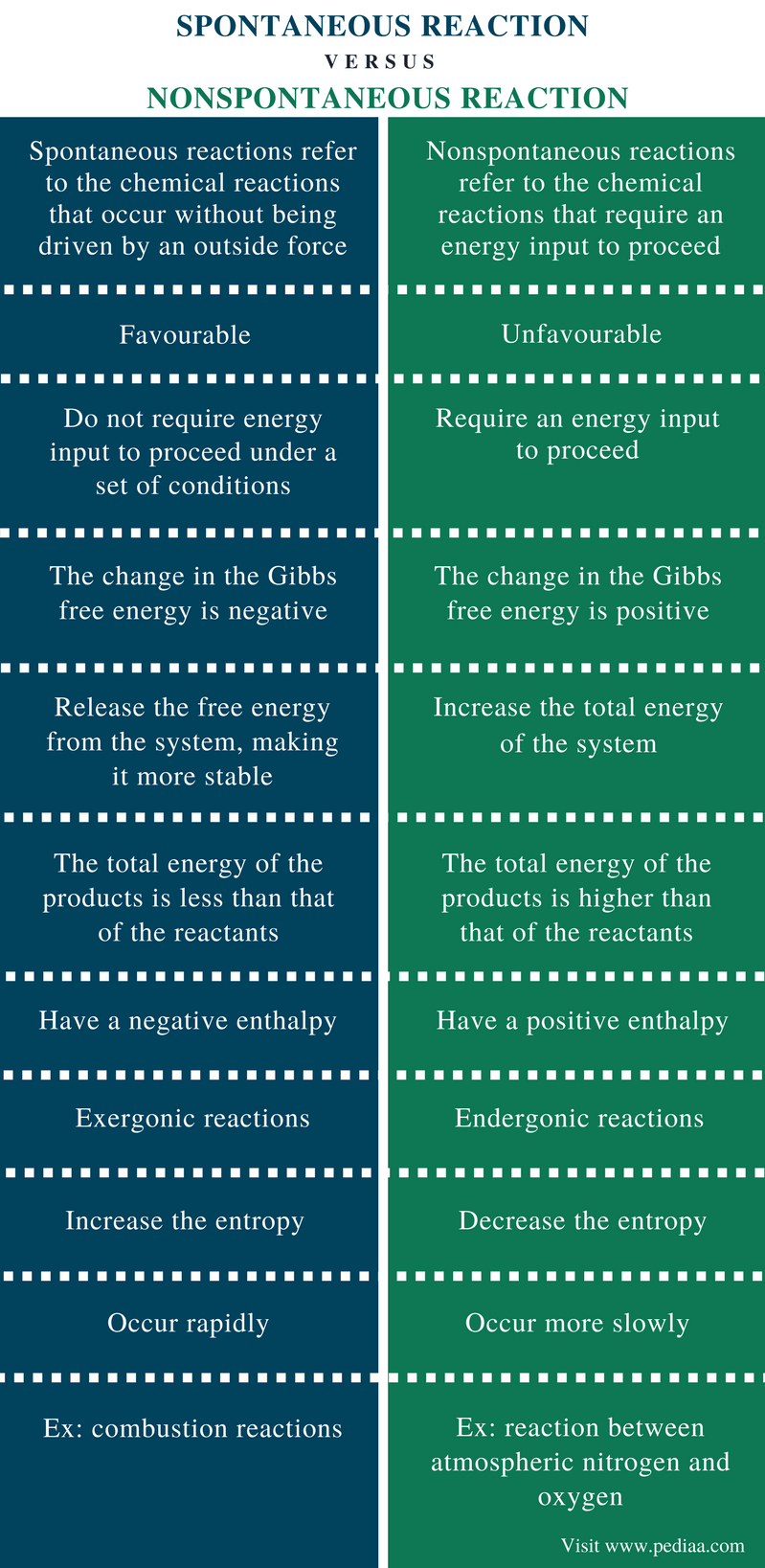 |
| Difference Between Spontaneous And Nonspontaneous Reactions Definition Thermodynamics Examples Similarities And Differences |
 |
| Free Energy Endergonic Vs Exergonic Reactions Article Khan Academy |
 |
| Solved 6 2 Metabolic Reactions And Energy Release Energy Chegg Com |
Posting Komentar untuk "are endergonic reactions spontaneous"